Scientists to explore metal−nanotube interactions in the TEM
January 17, 2012 - The full understanding of the interaction of transition metals and carbon nanotubes is of great importance for the development of new advanced materials, e.g. in electronic components and catalysts. A group of researchers from the Universities of Ulm (Germany) and Nottingham (UK) as well as the company Zeiss (Germany) has now for the first time studied the interaction of the transition metals W, Re and Os with the interior of CNTs by imaging at the single-atom level in direct space and real time using LV-AC-HRTEM combined with DFT simulations.
The full understanding of the nature of the bond between carbon nanotubes and transition metals is increasingly a focus of nano-materials science today.[1-5] This is due to the promising opportunities for the applications of the metal SWNT heterostructures in catalysis,[6-9] hydrogen storage,[10] and microelectronics.[11-15] High-resolution transmission electron microscopy (HRTEM) developed with the advent of systematic investigations at low voltages to a reference method for studies at atomic resolution. New research results can now show for the first time the complex nature of the nanotube-metal bond with atomic resolution in real time.[16-21]
From the atoms to the edge
The outer surface and the inner surface of carbon nanotubes have so far been investigated to different degrees.[22] The chemistry of the CNT's inside has remained largely unexplored. Now, a team of scientists has investigated the behavior of the three transition metals, W, Re and Os, after they had encapsulated them in the nanotubes.[16-21] The electron beam enabled a detailed study of the metal clusters and, at the same time, provided energy in the form of the kinetic energy of the electron beam for possible chemical transformations in the sample.[23,24]
At a standard energy of 200–300 kV, which is typically used in HRTEM experiments, damage to the nanotube structure occurs too quickly and it cannot be investigated at the atomic level.[25,26] By reducing the energy of the electron beam to 80 kV or less, the maximal transferable energy from an incident electron to a carbon atom is below the minimum energy required for a direct removal of a carbon atom from a nanotube making a detailed atomic-scale investigation possible.
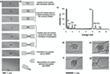
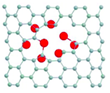
The metal atoms coordinated to the inner surface of the nanotube were found to increase the reactivity of the CC bonds near the clusters and lead to the removal of the carbon atoms (Figure 1). The coupling of the outermost metal atoms with the SWNT (Figure 2) and the redistribution of electrons between nanotubes and metal can stretch and thus weaken the CC bonds of the ligand molecules.[27] Out of the three elements, Os activates aromatic molecules to a greater degree than W or Re.[28]
The scientists then also analyzed the complete cycle of chemical reactions until the metal atoms are released from the MWNT. A number of previous reports has already shown a significantly stronger bond of metal atoms to vacancy defects in graphene compared to an untouched graphene structure.[29-33] Transition metals appeared to bond more strongly with dangling carbon bonds than with the π-electron system of an intact MWNT.[34] (Figure 3) As they later discovered, the model could also explain the observed formation of a cap in the process. Their experimental AC-HRTEM observations showed that from one point on, the nanotube defect largely reconstructs until a fully sealed cap with no dangling bonds and six pentagons (required for a closed cap topology) is formed (Figure 1g). Once the metal cluster has separated from the nanotube, no further transformations could take place in the SWNT structure.
At 80 kV, the research relies on observations made with the CS-corrected FEI Titan 80-300 TEM and the Zeiss Libra 200MC TEM. For 20 kV AC HRTEM experiments, special modifications of the corrector and base structure of the Zeiss Libra 200MC TEM were made as part of the SALVE project.[35] For instance, an improved monochromator (0.15 eV) was made to shift the information limit. Moreover, the Contrast improved through loss-free energy filtering (5 eV). The results were published on January 17 in the Journal of the American Chemical Society.
"High-resolution transmission electron microscopy (HRTEM) is rapidly developing into an excellent local probe tool for studying chemical reactions in nanotubes by mapping transformations in direct space and in real time down to the individual atom level," said SALVE Prof. Ute Kaiser, head of the SALVE project.
The recently demonstrated ability for HR-TEM to map the electron density distribution in carbon- and nitrogen-containing structures[36] is to be extended to organometallic systems in the future in order to further understand the complexity of the nanotube-metal bond at the subatomic level.
Resource: Zoberbier, T., Chamberlain, T. W., Biskupek, J., Kuganathan, N., Eyhusen, S., Bichoutskaia, E., Kaiser, U. & Khlobystov, A. N. (2012). Interactions and reactions of transition metal clusters with the interior of single-walled carbon nanotubes imaged at the atomic scale. Journal of the American Chemical Society, 134, 3073-3079, doi: 10.1021/ja208746z, [PDF], see also the [Supporting information].
-
Yang, S. H., Shin, W. H., Lee, J. W., Kim, S. Y., Woo, S. I., & Kang, J. K. (2006). Interaction of a transition metal atom with intrinsic defects in single-walled carbon nanotubes. The Journal of Physical Chemistry B, 110(28), 13941-13946.
-
Zhuang, H. L., Zheng, G. P., & Soh, A. K. (2008). Interactions between transition metals and defective carbon nanotubes. Computational Materials Science, 43(4), 823-828.
-
Valencia, H., Gil, A., & Frapper, G. (2010). Trends in the adsorption of 3d transition metal atoms onto graphene and nanotube surfaces: a DFT study and molecular orbital analysis. The Journal of Physical Chemistry C, 114(33), 14141-14153.
-
Inntam, C., & Limtrakul, J. (2010). Adsorption of M species and M2 dimers (M= Cu, Ag, and Au) on the pristine and defective single-walled carbon nanotubes: a density functional theory study. The Journal of Physical Chemistry C, 114(49), 21327-21337.
-
Chen, Y. K., Liu, L. V., Tian, W. Q., & Wang, Y. A. (2011). Theoretical Studies of Transition-Metal-Doped Single-Walled Carbon Nanotubes. The Journal of Physical Chemistry C, 115(19), 9306-9311.
-
Tian, W. Q., Liu, L. V., & Wang, Y. A. (2006). Electronic properties and reactivity of Pt-doped carbon nanotubes. Physical Chemistry Chemical Physics, 8(30), 3528-3539.
-
Kong, K. J., Choi, Y., Ryu, B. H., Lee, J. O., & Chang, H. (2006). Investigation of metal/carbon-related materials for fuel cell applications by electronic structure calculations. Materials Science and Engineering: C, 26(5), 1207-1210.
-
Pan, X., & Bao, X. (2008). Reactions over catalysts confined in carbon nanotubes. Chemical Communications, (47), 6271-6281.
-
Castillejos, E., Debouttière, P. J., Roiban, L., Solhy, A., Martinez, V., Kihn, Y., Ersen, O., Phillipot, K., Chaudret, B., & Serp, P. (2009). An efficient strategy to drive nanoparticles into carbon nanotubes and the remarkable effect of confinement on their catalytic performance. Angewandte Chemie International Edition, 48(14), 2529-2533.
-
Yildirim, T., & Ciraci, S. (2005). Titanium-decorated carbon nanotubes as a potential high-capacity hydrogen storage medium. Physical Review Letters, 94(17), 175501.
-
Dong, X., Lau, C. M., Lohani, A., Mhaisalkar, S. G., Kasim, J., Shen, Z., Ho, X., Roger, J. A., & Li, L. J. (2008). Electrical Detection of Femtomolar DNA via Gold‐Nanoparticle Enhancement in Carbon‐Nanotube‐Network Field‐Effect Transistors. Advanced Materials, 20(12), 2389-2393.
-
Nemec, N., Tománek, D., & Cuniberti, G. (2006). Contact dependence of carrier injection in carbon nanotubes: an ab initio study. Physical Review Letters, 96(7), 076802.
-
Palacios, J. J., Pérez-Jiménez, A. J., Louis, E., SanFabián, E., & Vergés, J. A. (2003). First-principles phase-coherent transport in metallic nanotubes with realistic contacts. Physical Review Letters, 90(10), 106801.
-
Yang, C. K., Zhao, J., & Lu, J. P. (2004). Complete spin polarization for a carbon nanotube with an adsorbed atomic transition-metal chain. Nano Letters, 4(4), 561-563.
-
Rodríguez-Manzo, J. A., Banhart, F., Terrones, M., Terrones, H., Grobert, N., Ajayan, P. M., Sumpter, B. G., Meunier, V., Wang, M., Bando, Y., & Golberg, D. (2009). Heterojunctions between metals and carbon nanotubes as ultimate nanocontacts. Proceedings of the National Academy of Sciences, 106(12), 4591-4595.
-
Nakamura, E., Koshino, M., Saito, T., Niimi, Y., Suenaga, K., & Matsuo, Y. (2011). Electron microscopic imaging of a single group 8 metal atom catalyzing C–C bond reorganization of fullerenes. Journal of the American Chemical Society, 133(36), 14151-14153.
-
Koshino, M., Tanaka, T., Solin, N., Suenaga, K., Isobe, H., & Nakamura, E. (2007). Imaging of single organic molecules in motion. Science, 316(5826), 853-853.
-
Koshino, M., Niimi, Y., Nakamura, E., Kataura, H., Okazaki, T., Suenaga, K., & Iijima, S. (2010). Analysis of the reactivity and selectivity of fullerene dimerization reactions at the atomic level. Nature Chemistry, 2(2), 117-124.
-
Chuvilin, A., Bichoutskaia, E., Gimenez-Lopez, M. C., Chamberlain, T. W., Rance, G. A., Kuganathan, N., Biskupek, J., Kaiser, U., & Khlobystov, A. N. (2011). Self-assembly of a sulphur-terminated graphene nanoribbon within a single-walled carbon nanotube. Nature Materials, 10(9), 687-692.
-
Chamberlain, T. W., Meyer, J. C., Biskupek, J., Leschner, J., Santana, A., Besley, N. A., Bichoutskaia, E., Kaiser, U., & Khlobystov, A. N. (2011). Reactions of the inner surface of carbon nanotubes and nanoprotrusion processes imaged at the atomic scale. Nature Chemistry, 3(9), 732-737.
-
Chuvilin, A., Kaiser, U., Bichoutskaia, E., Besley, N. A., & Khlobystov, A. N. (2010). Direct transformation of graphene to fullerene. Nature Chemistry, 2(6), 450-453.
-
Tasis, D., Tagmatarchis, N., Bianco, A., & Prato, M. (2006). Chemistry of carbon nanotubes. Chemical Reviews, 106(3), 1105-1136.
-
Williams, D. B., & Carter, C. B. Transmission electron microscopy: a textbook for materials science. 2009.
-
McKinley Jr, W. A., & Feshbach, H. (1948). The coulomb scattering of relativistic electrons by nuclei. Physical Review, 74(12), 1759.
-
Smith, B. W., & Luzzi, D. E. (2001). Electron irradiation effects in single wall carbon nanotubes. Journal of Applied Physics, 90(7), 3509-3515.
-
Zobelli, A., Gloter, A., Ewels, C. P., Seifert, G., & Colliex, C. (2007). Electron knock-on cross section of carbon and boron nitride nanotubes. Physical Review B, 75(24), 245402.
-
Muetterties, E. L., Bleeke, J. R., Wucherer, E. J., & Albright, T. (1982). Structural, stereo-chemical, and electronic features of arene-metal complexes. Chemica l Reviews, 82(5), 499-525.
-
Ding, F., & Harman, W. D. (2004). Stereoselective tandem 1, 4-addition reactions for benzenes: A comparison of Os (II), Re (I), and W (0) systems. Journal of the American Chemical Society, 126(42), 13752-13756.
-
Krasheninnikov, A. V., Lehtinen, P. O., Foster, A. S., Pyykkö, P., & Nieminen, R. M. (2009). Embedding transition-metal atoms in graphene: structure, bonding, and magnetism. Physical Review Letters, 102(12), 126807.
-
Gan, Y., Sun, L., & Banhart, F. (2008). One‐and Two‐Dimensional Diffusion of Metal Atoms in Graphene. Small, 4(5), 587-591.
-
Chan, K. T., Neaton, J. B., & Cohen, M. L. (2008). First-principles study of metal adatom adsorption on graphene. Physical Review B, 77(23), 235430.
-
Anton, R., & Schneidereit, I. (1998). In situ TEM investigations of dendritic growth of Au particles on HOPG. Physical Review B, 58(20), 13874.
-
Rodríguez-Manzo, J. A., Cretu, O., & Banhart, F. (2010). Trapping of metal atoms in vacancies of carbon nanotubes and graphene. ACS nano, 4(6), 3422-3428.
-
Meyer, J. C., Kurasch, S., Park, H. J., Skakalova, V., Künzel, D., Groß, A., Chuvilin, A., Algara.Siller, G., Roth, S., Iwasaki, T., Starke, U., Smet, J., & Kaiser, U. (2011). Experimental analysis of charge redistribution due to chemical bonding by high-resolution transmission electron microscopy. Nature Materials, 10(3), 209-215.
-
Kaiser, U., Biskupek, J., Meyer, J. C., Leschner, J., Lechner, L., Rose, H., Stöger-Pollach, M., Khlobystov, A. N., Hartel, P., H. Müller, Haider, M., Eyhusen, S., & Benner, G. (2011). Transmission electron microscopy at 20kV for imaging and spectroscopy. Ultramicroscopy, 111(8), 1239-1246.
-
Meyer, J. C., Chuvilin, A., Algara-Siller, G., Biskupek, J., & Kaiser, U. (2009). Selective sputtering and atomic resolution imaging of atomically thin boron nitride membranes. Nano Letters, 9(7), 2683-2689.