SALVE microscopists to explore bond breaking and forming of individual diatomic molecules
January 17, 2020 - A new study of a research team from Germany and the UK sheds light on the dynamics of metallic bonds between two atoms in real time and in direct space from examining the correlated motion of a two-atomic molecule directly in the SALVE TEM. It has been shown for the first time that the Re2 molecules, moving freely along the SWNT at room temperature, continuously change the binding order between one, two and four.
Since 1964,[1, 2] the global effort in understanding the nature of the metal–metal (M–M) bond has been growing strongly. Metal-metal bond (MM)[3] describing attractive interactions between metal centres were recently detected in the noble gas matrices under cryogenic conditions.[4] Also heteronuclear diatom molecule hasve been proven experimentally.[5] However, a central challenge is still to determine the order of MM bonds. According to theoretical calculations,[6] variation between the order of one and five has a strong influence on the physical and chemical properties of the molecules.[3]
Previously, to determine the binding order of molecules, spectroscopic methods such as nuclear magnetic resonance, infrared spectroscopy, UV spectroscopy and X-ray diffraction were mainly used.[7, 8] In the new study, the researchers have now presented results in which they have studied the bond order of dirhenium molecules using single-walled carbon nanotubes (SWNTs) as nano test tubes with transmission electron microscopy (TEM). This method has the advantage that the structure and dynamics on the level of the individual atom can be mapped in real time and in real space.
First record of the breakdown and formation of individual diatomic molecules
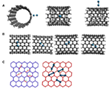
The electron beam of the SALVE³ TEM[9, 10] enabled the stimulated dynamics of dirhenium molecules to be recorded with the higher spatial resolution of a chromatic and spherical aberration (Cc/Cs)-corrected TEM (Video 1). Figure 1 shows the first stage of the Video 1 from 0 to 87 s.
0 s: The data analysis showed at the beginning the presence of a Re2 molecule on the upper SWNT. A van der Waals gap (vdW) of 0.3 nm is formed by the two parallel SWNTs with different chirality. After a period of time, the two Re atoms of the Re2 dimer begin to vibrate, distorting their circular shapes into ellipses and stretching the bond. When the bond length reached a value that exceeded the sum of the atomic radii, the bond snapped open, creating two independent atoms. A little later, the atoms reconnected and reformed the Re2 molecule.
After 50 s: An intact SWNT is impermeable to almost all atoms and ions except H+,[11] but a vacancy defect on the SWNT could provide a channel for the penetration of atoms. In the following 20 s, the entire dirhenium molecule exits and begins to drift along the outer surface of the host SWNT, which is caused by stronger binding of the metal to an external defect, in good agreement with simulations.[12]
72 - 85 s: The dirhenium molecule drifts along the outer surface of the host SWNT and then slides into the one-dimensional vdW gap (yellow arrows in Figure 1B). The simulated structure is shown in Figure 2B with a Re-Re bond length of 0.22 nm.
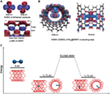
89 s: The vdW gap between the two parallel SWNTs in the initial state of the second stage is approximately 0.32 nm (± 0.010 nm), which agrees with the theoretical prediction of the Lennard-Jones model.[13] This vdW gap limits Re2 and provides a one-dimensional channel in the vacuum, which limits Re2 translation and rotation.
87 to 94 s: After the dirhenium molecule has moved into the vdW gap, the contrast of the Re atoms shows unusual symmetrically elongated features, which indicates that the Re atoms are delocalized in the time frame of a single exposure (0.5) (Figure 2B). The centre distance of the elongated points is 0.36 nm, which is slightly longer than the Re-Re single bond in compounds.[14] This can be attributed to an oscillation of the Re atoms with an amplitude of ± 0.04 nm.
94 s: Re2 breaks into two unbound Re atoms with a distance of 0.58 nm. After the separation, the contrast between two Re atoms is no longer extended. This observation shows that as soon as the length of the Re-Re bond exceeds that of a single bond, the molecule can be in an excited state, which manifests itself in a large amplitude of vibration. The bond breaks beyond a critical distance. Re2 acts like a harmonic oscillator which is pumped with the kinetic energy transferred from the 80 keV electrons to the Re atom until it is large enough to overcome the barrier of dissociation of the Re-Re bond.
94 to 97 s: The two Re atoms move freely in the one-dimensional vdW gap until they collide after 97 s and recombine to Re2. The bond distance of the reformed Re2 decreases from 0.30 (97 s) to 0.23 nm (132 s). The contrast of two Re atoms at 132 s is almost round with a distance of 0.23 nm, which indicates that the two Re atoms are again bound by a quadruple bond. This reformed dirhenium molecule shows the same properties as before and moves in the following 34 s between different orientations in the one-dimensional vdW gap, similar to the behaviour within and on the SWNT. The scientists had previously shown that such free Re atoms are chemically active towards the SWNT.[15] As a result, the carbon lattice is restructured after 97 s.
200 s: Re2 dissociates again and recombines after 212 s. This dirhenium molecule moves in the vdW gap in the following 181 s and merges into the large Re cluster in the lower SWNT.
Ute Kaiser says "We use the electron beam as an energy source and as an imaging probe for our investigations at the same time and understand the bonding characteristic between two metal atoms. We hope that now further systematic investigation of metal bonds between different atoms can be performed to determine their most stable configuration and electronic structure."
For instance, a search for possible stable bond configurations within the SWNT already revealed the existence of a single standing mode with a Re-Re bond length of 0.22 nm, in which Re2 binds to all six carbon atoms of a single hexagon, which is influenced by the curvature of the nanotube wall.
The binding energy of this configuration is -165 kJ / mol (-1.71 eV), based on the separated dirhenium molecule and SWNT. At higher energy several configurations are geometrically and energetically similar (Figure 3).
The atomic Re-orbitals and the occupied and deep-lying unoccupied orbitals of Re2 are shown in Figure 4. In order to understand the observed bonding configurations, it is instructive to study the spatial extent of the molecular orbitals involved in bonding. Due to the overlap of the carbon atoms, the bond will end up being more unstable than the side bond. On the outside of the SWNT, the rhenium dimer meets the convex curvature of the nanotube, and two carbon atoms are further away from the dimer than the other four. Thus, the transport seems to be largely influenced by the curvature of the SWNT wall. It can be assumed that the wider nanotube with a lower degree of curvature restricts the possible binding modes less and that the outside of the tube behaves more similarly.
Using our experimental observations in conjunction with theoretical modelling, Figure 5C shows a plausible energy profile for the movement of Re2 along the nanotube. Under our conditions, the electron beam transmits energy in the order of seconds to excite Re2, from which the molecule can relax into neighboring energy minima (Figure 5C).
A basic assumption for the study is the extreme high specimen resolution as well as the high contrast in the HRTEM image achievable through the new technique of chromatic and spherical aberration correction in a low-voltage TEM (SALVE). Numerous new results have already been obtained. An unexpected bonding state where the Re-Re bond order is less than one, accompanied with large amplitude of vibration observed just before the bond dissociation, indicates the existence of previously unattended bonding states between metal atoms.
Resource: Cao, K., Skowron, S. T., Biskupek, J., Stoppiello, C. T., Leist, C., Besley, E., Khlobystov, A. N. & Kaiser, U. (2020). Imaging an unsupported metal–metal bond in dirhenium molecules at the atomic scale. Science Advances, 6, eaay5849, doi: 10.1126/sciadv.aay5849.
-
F. A. Cotton, N. F. Curtis, C. B. Harris, B. F. G. Johnson, S. J. Lippard, J. T. Mague, W. R. Robinson, J. S. Wood, Mononuclear and polynuclear chemistry of rhenium (III): Its pronounced homophilicity. Science 145, 1305–1307 (1964).
-
F. A. Cotton, Metal-metal bonding in [Re2X8]2−Ions and other metal atom clusters. Inorg. Chem. 4, 334–336 (1964).
-
F. A. Cotton, Carlos A. Murillo, Richard A. Walton, Multiple bonds between metal atoms. (Springer Science and Business Media, 2005).
-
Z. Hu, B. Shen, Q. Zhou, S. Deosaran, J. R. Lombardi, D. M. Lindsay, W. Harbich, Raman spectra of mass-selected vanadium dimers in argon matrices. J. Chem. Phys. 95, 2206–2209 (1991).
-
L. R. Liu, J. D. Hood, Y. Yu, J. T. Zhang, N. R. Hutzler, T. Rosenband, K.-K. Ni, Building one molecule from a reservoir of two atoms. Science 360, 900–903 (2018).
-
L. Gagliardi, B. O. Roos, Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond. Nature 433, 848–851 (2005).
-
T. Nguyen, A. D. Sutton, M. Brynda, J. C. Fettinger, G. J. Long, P. P. Power, Synthesis of a stable compound with fivefold bonding between two chromium(I) centers. Science 310, 844–847 (2005).
-
K. A. Kreisel, G. P. A. Yap, O. Dmitrenko, C. R. Landis, K. H. Theopold, The shortest metal-metal bond yet: Molecular and electronic structure of a dinuclear chromium diazadiene complex. J. Am. Chem. Soc. 129, 14162–14163 (2007).
-
M. Linck, P. Hartel, S. Uhlemann, F. Kahl, H. Müller, J. Zach, M. Haider, M. Niestadt, M. Bischoff, J. Biskupek, Z. Lee, T. Lehnert, F. Börrnert, H. Rose, U. Kaiser, Chromatic aberration correction for atomic resolution TEM imaging from 20 to 80 kV. Phys. Rev. Lett. 117, 076101 (2016).
-
S. T. Skowron, T. W. Chamberlain, J. Biskupek, U. Kaiser, E. Besley, A. N. Khlobystov, Chemical reactions of molecules promoted and simultaneously imaged by the electron beam in transmission electron microscopy. Acc. Chem. Res. 50, 1797–1807 (2017).
-
S. Hu, M. Lozada-Hidalgo, F. C. Wang, A. Mishchenko, F. Schedin, R. R. Nair, E. W. Hill, D. W. Boukhvalov, M. I. Katsnelson, R. A. W. Dryfe, I. V. Grigorieva, H. A. Wu, A. K. Geim, Proton transport through one-atom-thick crystals. Nature 516, 227–230 (2014).
-
A. N. Andriotis, M. Menon, G. Froudakis, Catalytic action of Ni atoms in the formation of carbon nanotubes: A molecular dynamics study. Phys. Rev. Lett. 85, 3193–3196 (2004).
-
C.-H. Sun, G.-Q. Lu, H.-M. Cheng, Simple approach to estimating the van der Waals interaction between carbon nanotubes. Phys. Rev. B 73, 195414 (2006).
-
O. Cretu, A. V. Krasheninnikov, J. A. Rodríguez-Manzo, L. Sun, R. M. Nieminen, F. Banhart, Migration and localization of metal atoms on strained graphene. Phys. Rev. Lett. 105, 196102 (2010).
-
T. W. Chamberlain, J. C. Meyer, J. Biskupek, J. Leschner, A. Santana, N. A. Besley, E. Bichoutskaia, U. Kaiser, A. N. Khlobystov, Reactions of the inner surface of carbon nanotubes and nanoprotrusion processes imaged at the atomic scale. Nat. Chem. 3, 732–737 (2011).