From Point Defects in Graphene to Two-Dimensional Amorphous Carbon.
March 09, 2011 – A team of scientists from Finland and Germany has studied the transformation of graphene into two-dimensional amorphous carbon. The observations, along with first-principles calculations, provide new insights to the bonding behavior of carbon and the dynamics of defects in graphene. The created domains possess a band gap, which may open new possibilities for engineering graphene-based electronic devices.
Aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM) has recently allowed atomic-resolution imaging of regular carbon nanostructures and identification of defects in these materials [1,2]. Earlier experiments on curved carbon nanosystems [3,4] had shown that they avoid under-coordinated atoms under irradiation at high temperatures via vacancy migration and coalescence. The arrangement of other polygons can be geometrically deduced via the isolated pentagon rule (IPR) [5-7] and Euler’s theorem [8]. In fullerenes [5] and carbon nanohorns [6], nonhexagonal rings usually lead to the development of nonzero curvature. However, despite the recent advances, the precise microscopic picture of the response of graphene to electron irradiation remained incomplete.
Now the scientists made a new study with a spherical aberration-corrected FEI Titan 80–300 TEM, equipped with an objective-side image corrector. The microscope was operated at low voltage for HRTEM imaging, and at 300 kV for defect production, above the knock-on threshold of graphene [9,10]. The atomic scale study with underpinned with Density-functional theory (DFT) calculations.
Building a two-dimensional amorphous membrane
"This is the first time that anyone has systematically tried to transform and observe the transformation of a two-dimensional material from the crystalline to the amorphous state with that high precision.” says Jannik Meyer from the group of Electron Microscopy for Materials Science (EMMS) at Ulm University.
The scientists reported now the transformation of graphene into a two-dimensional random arrangement of polygons. Until now, experiments on graphene [2] reported only the development of holes. Only theoretical studies had also predicted the appearance of small holes or formation of haeckelitelike configurations [11] or dislocations [12].
Video 1: The transformation from a crystalline to an amorphous two-dimensional material created by a high-dose exposure of graphene to 100 kV electrons.
They show that the transformation are driven by two simple mechanisms: atom ejection and bond rotation. They also discovered unexpected configurations, such as stable carbon tetragons [13] which appear upon linear arrangement of divacancies. Contrary to the expectations, the material did not collapse but remained stable.
The challenge of studying undercoordinated systems
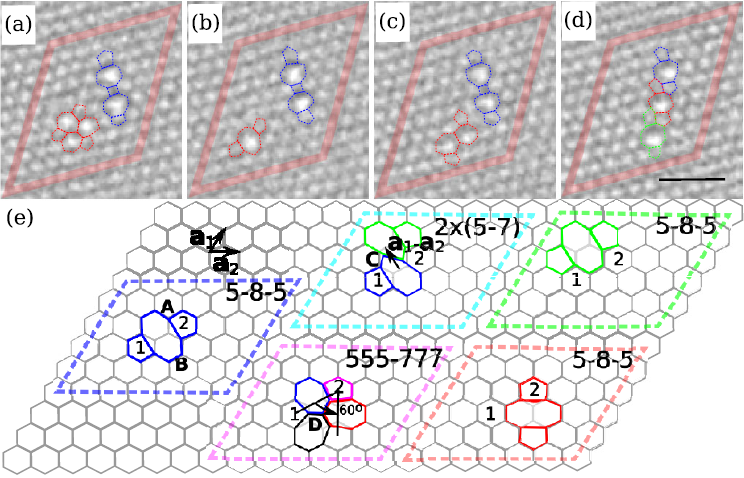
To understand the mechanisms of the transformation, the scientists separated them by varying the electron beam energy. They created initial damage in a graphene sheet by a brief 300 kV irradiation. Then they studied them at 80 kV, where pristine graphene remains stable, but under-coordinated atoms can be still be ejected (Td for a sp2—bonded C is about 18–20 eV [9,10]). Also bond reorganization is still possible, as activation energies for bond rotations in sp2—bonded carbon structures are in the range of 4–10 eV [14-16] (Figs. 1(a) and 1(b)) resulting in the formation of the Stone-Wales defect [17], and lower votages are desired to observe defective graphene. Furthermore, defect structures, such as divacancies, can be produced (Figure 1).
A brief exposure to a 300 kV beam (dose ~107 e-/nm2) creates mainly mono-vacancies, which then convert to divacancies. Because atom ejection occurs at random positions, vacancies initially appear randomly in the area exposed to the electron beam. During the image sequence at 80 kV the vacancies start to migrate. Each migration step is initiated by a single electron impact from which the atom obtains energy slightly below Td. In other words, electron irradiation provides the activation energy to drive the system from a local energy minimum into another one.
Figures 2 shows a collapse of linearly clustered defects into an apparently less defective structure with a dislocation dipole. This effect has earlier been predicted for graphene [12]. During continued irradiation, the scientists now observed the formation of a cluster of rotated hexagons surrounded by a chain of alternating pentagons and heptagons. Such configurations, rotated by 30° with respect to the original lattice and matched by pentagons and heptagons to the zigzag lattice direction, seem to be the preferred way to incorporate missing atoms in the graphene structure. Because of the matching numbers of pentagons and heptagons—and hence cancellation of negative and positive curvature—these structures remain flat.
To understand the transformations, the scientists calculated the formation energy of the experimentally observed structures using DFT. [18-23] The lowest-energy tetra-vacancy (four missing atoms) can be created by combining two V2 (5555-6-7777) divacancies, whereas the hexavacancy (six missing atom) requires three of these defects (Figure 3). Remarkably, these configurations have the lowest formation of any reported vacancy structures with equal number of missing atoms in graphene. A hole with six missing atoms has formation energy of ~6 × 3.15 eV, while for a dislocation and haeckelitelike structure values of ~6 × 3.72 eV and ~6 × 2.64 eV have been reported earlier [12]. The rotated-hexagon defects spawn the family of lowest-energy multivacancies in graphene.
The richness of carbon continues to surprise
The new material can be termed amorphous graphene might be interesting for applications but again provides new insides in fundamental physics. The scientists showed that in contrast to the zigzag-oriented divacancy [24], the rotated-hexagon defects open a band gap in graphene, as can be seen from the density of states in Fig. 3. The calculated band gap is in the order of 200 meV. More advanced DFT methods shall be applied to further investigate the new material in the future [25].
“Due to increased reactivity amorphous two-dimensional carbon may also be exploited for functionalization of graphene.” Ute Kaiser, says. “Clearly, despite the large amount of research, the richness of carbon chemistry continues to provide surprises. The understanding of the driving force for the transformations is also an essential step to control radiation damage which is a central goal of every electron microscopist.”
Resource: Kotakoski, J., Krasheninnikov, A. V., Kaiser, U. A. & Meyer, J. C. (2012). From Point Defects in Graphene to Two-Dimensional Amorphous Carbon. Phys. Rev. Lett., 106, 105505, doi: 10.1103/PhysRevLett.106.105505, [PDF], see also the [Supporting information].
Girit, Ç. Ö., Meyer, J. C., Erni, R., Rossell, M. D., Kisielowski, C., Yang, L., Park, C.-H., Crommie, M. F., Cohen, M. L., Louie, S. G., & Zettl, A. (2009). Graphene at the edge: stability and dynamics. Science, 323(5922), 1705-1708.
Warner, J. H., Rümmeli, M. H., Ge, L., Gemming, T., Montanari, B., Harrison, N. M., Büchner, B., & Briggs, G. A. D. (2009). Structural transformations in graphene studied with high spatial and temporal resolution. Nature nanotechnology, 4(8), 500-504.
Sun, L., Banhart, F., Krasheninnikov, A. V., Rodriguez-Manzo, J. A., Terrones, M., & Ajayan, P. M. (2006). Carbon nanotubes as high-pressure cylinders and nanoextruders. Science, 312(5777), 1199-1202.
Sun, L., Krasheninnikov, A. V., Ahlgren, T., Nordlund, K., & Banhart, F. (2008). Plastic deformation of single nanometer-sized crystals. Physical review letters, 101(15), 156101.
Kroto, H. W. (1987). The stability of the fullerenes Cn, with n= 24, 28, 32, 36, 50, 60 and 70. Nature, 329(6139), 529-531.
Iijima, S., Yudasaka, M., Yamada, R., Bandow, S., Suenaga, K., Kokai, F., & Takahashi, K. (1999). Nano-aggregates of single-walled graphitic carbon nano-horns. Chemical Physics Letters, 309(3), 165-170.
Schmalz, T. G., Seitz, W. A., Klein, D. J., & Hite, G. E. (1988). Elemental carbon cages. Journal of the American Chemical Society, 110(4), 1113-1127.
Terrones, H., & Mackay, A. L. (1992). The geometry of hypothetical curved graphite structures. Carbon, 30(8), 1251-1260.
Banhart, F. (1999). Irradiation effects in carbon nanostructures. Reports on progress in physics, 62(8), 1181.
Smith, B. W., & Luzzi, D. E. (2001). Electron irradiation effects in single wall carbon nanotubes. Journal of Applied Physics, 90(7), 3509-3515.
Terrones, H., Terrones, M., Hernández, E., Grobert, N., Charlier, J. C., & Ajayan, P. M. (2000). New metallic allotropes of planar and tubular carbon. Physical review letters, 84(8), 1716.
Jeong, B. W., Ihm, J., & Lee, G. D. (2008). Stability of dislocation defect with two pentagon-heptagon pairs in graphene. Physical Review B, 78(16), 165403.
Legrand, Y. M., Van der Lee, A., & Barboiu, M. (2010). Single-crystal X-ray structure of 1, 3-dimethylcyclobutadiene by confinement in a crystalline matrix. Science, 329(5989), 299-302.
Meyer, J. C., Kisielowski, C., Erni, R., Rossell, M. D., Crommie, M. F., & Zettl, A. (2008). Direct imaging of lattice atoms and topological defects in graphene membranes. Nano letters, 8(11), 3582-3586.
Ewels, C. P., Heggie, M. I., & Briddon, P. R. (2002). Adatoms and nanoengineering of carbon. Chemical Physics Letters, 351(3), 178-182.
Li, L., Reich, S., & Robertson, J. (2005). Defect energies of graphite: density-functional calculations. Physical Review B, 72(18), 184109.
Stone, A. J., & Wales, D. J. (1986). Theoretical studies of icosahedral C 60 and some related species. Chemical Physics Letters, 128(5), 501-503.
Kresse, G., & Furthmüller, J. (1996). Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 6(1), 15-50.
Kresse, G., & Furthmüller, J. (1996). Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical review B, 54(16), 11169.
Blöchl, P. E. (1994). Projector augmented-wave method. Physical Review B, 50(24), 17953.
Perdew, J. P., Burke, K., & Ernzerhof, M. (1996). Generalized gradient approximation made simple. Physical review letters, 77(18), 3865.
Monkhorst, H. J., & Pack, J. D. (1976). Special points for Brillouin-zone integrations. Physical review B, 13(12), 5188.
Henkelman, G., Uberuaga, B. P., &Jónsson, H. (2000). A climbing image nudged elastic band method for finding saddle points and minimum energy paths. The Journal of chemical physics, 113(22), 9901-9904.
Lahiri, J., Lin, Y., Bozkurt, P., Oleynik, I. I., & Batzill, M. (2010). An extended defect in graphene as a metallic wire. Nature nanotechnology, 5(5), 326-329.
Appelhans, D. J., Lin, Z., & Lusk, M. T. (2010). Two-dimensional carbon semiconductor: Density functional theory calculations. Physical Review B, 82(7), 073410.
Orellana, W. (2009). Reaction and incorporation of H2 molecules inside single-wall carbon nanotubes through multivacancy defects. Physical Review B, 80(7), 075421.