Defect Influence on Lithium Crystallization in Graphene Layers Observed via TEM
July 18, 2024 - Despite extensive research focusing on improving performance such as energy and power density, lithium-ion batteries suffer from capacity loss and resistance increase, leading to both energy and power fade during usage. Researchers from the University and the Helmholtz Institute in Ulm, Germany, the Karlsruhe Institute of Technology, CEOS GmbH, the Helmholtz-Zentrum Dresden-Rossendorf, the University of Science and Technology of China in Hefei as well as the Qufu Normal University in China have now reported a new study of the behavior of nucleation and crystal formation during lithiation and delithiation at the atomic scale.
Numerous studies have been carried out to understand the failure mechanisms of lithium-ion batteries (LIBs) in order to improve their lifetime [1]. The fading of LIBs is a consequence of many factors [2], for example, lithium plating [3] and degradation of the positive electrode [4, 5]. Crystal defects have been proven to play a critical role in all these factors [6, 7]. Moreover, capacity loss and current leakage resulting from the decomposition of the electrodes and electrolyte are affected by the size and position of the defects [8]. However, defects do not always have a detrimental effect on LIB operation. The aliovalent substitution of Fe3+ for Zn2+ in ZnO anodes can lead to the formation of local defects such as cationic vacancies, which serve as potential sites for initial Li+ ion insertion, thus favoring the lithiation kinetics [9, 10]. Defects can also relieve strain between two phases during phase transformations in spinel LixMn2O4 cathode materials [11].
Among the crystal defects that can influence the functionality of battery materials are impurity atoms in substitutional and interstitial positions, as well as vacancies. These defects can appear at the stage of materials synthesis, during electrochemical cycling [12], high-temperature operation [13], or irradiation [10] when the battery is used in extreme environments, such as cosmic space.
While defects in inorganic materials and their impact on the de-/lithiation process have been the focus of recent research [14], the impact of defects in graphite, as the state-of-the-art anode material of today’s LIBs [15], has attracted significantly less attention. However, to understand the effects that crystal defects may have on battery electrode materials, atomic-scale imaging is one of the most suitable tools. Recently, the authors succeeded in imaging the lithiation and delithiation processes between sheets of bilayer graphene at the atomic scale by using chromatic and spherical aberration-corrected high-resolution transmission electron microscopy (CC/CS-corrected HRTEM) [16, 17]. These previous experiments also verified that lithiation can only occur between graphene sheets and not on top of or underneath single-layer graphene (for testing, lithium was placed additionally on top of single layers with no effect after biasing) [16]. The graphene layers also have to fully cover the lithium; larger holes result in a fast outgassing of the lithium into the vacuum [17]. The authors found that in bilayer graphene, lithium adopts an ultrathin and super dense close-packed phase with an in-plane lattice parameter of 3.1 Å, not the typical LiC6 phase observed after intercalation of lithium cations into graphite. In fact, the presence of such superdense lithium appears feasible only due to the ability of graphene to sufficiently expand in the vertical direction, which is suppressed in graphite owing to the required mechanical deformation of a larger number of graphene sheets. Nonetheless, an in-depth understanding of the underlying processes might help to better understand the lithiation and delithiation processes occurring in graphite, while simultaneously providing guidelines for further improved capacities in advanced active materials such as nanostructured carbons that provide sufficient space in their crystal structures.
In this study, the authors extend previous work with a detailed TEM analysis supported by electron energy loss spectroscopy and density functional theory calculations. They focus on the role that atomic defects (such as carbon vacancies in graphene and foreign oxygen atoms in the growing lithium crystal) can play during the cycling between graphene layers. They observed how ultrathin lithium crystals form and grow, and how impurities inside the lithium crystal can affect the delithiation process. The resulting insights could be crucial for a deeper understanding of the role of defects during charging and discharging processes in LIBs.
The authors report on the evaluation of data obtained for bi- and trilayer graphene to extend the system further toward graphite and find that the behavior of lithiation and delithiation is the same for both bi- and trilayer devices. The schematic setup in Figure 1, where the graphene layers are placed freely hanging over the holes in the Si3N4 membrane on the specially designed TEM sample carrier chip, is explained in more detail in [17]. However, the de-/lithiation experiments shown here were performed in situ in the CC/CS SALVE III instrument operated at 80 keV [18].
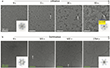
Next, in Figure 2, the authors present a sequence of images recorded during the first-cycle lithiation process (bilayer system) and a subsequent delithiation process (trilayer system). The Li crystals grow with faceted shapes during lithiation (Figure 2a), while during delithiation they do not simply shrink in shape but transform into unfaceted irregular structures (Figure 2b). Before the lithiation starts (Figure 2a), the pristine graphene is rather free of contamination. The inserted Fourier transform pattern confirms the presence of graphene (lattice constant of a = 2.46 Å). Already after 5 seconds of cycling, many Li nucleation seeds are formed and identified by their dark contrast (one dark patch is marked by the white arrow), with their density estimated as approximately 0.02 nm−2. As lithiation progresses further, the Li seeds grow into larger ultrathin crystalline plates with faceted shapes (most of them have a triangular shape) and merge with each other. The arrows in Figure 2a mark the same Li crystal at different times of the lithiation. The inset in the image recorded at t = 93 s shows the corresponding Fourier transform where only the second-order reflections of the Li phase [17] can be seen; however, the first-order reflections are missing. In the work presented here, the Li crystals nucleated inside the field of view, so their behavior in the early stages of lithiation, such as nucleation, growth, and coalescence of Li nanocrystals, could be directly observed and therefore better understood. Figure 2b shows an overview of TEM images of a delithiation process in a trilayer-graphene system.
Next, Figure 3a shows a series of CC/CS-corrected HRTEM images recorded during another lithiation experiment inside bilayer graphene, where the graphene lattice is removed by Fourier filtering in each image. The removal of the regular crystal lattice reflection of graphene has the positive side effect that lattice defects in graphene are recognized very easily as additional contrast. Before the lithiation starts, no Li crystal lattice can be observed. However, the authors detect dark spots (two examples are marked with white arrows) that were later identified as single vacancies in the graphene lattice. Already a few Li nanocrystals are visible. As the lithiation proceeds, the crystals grow with different orientations and then merge into one single crystal at t = 145 s. This can be easily seen as the crystals are color-coded according to their orientation [19]. The corresponding colored mask can be seen in the inserted FFT (fast Fourier transform) in the very right image in Figure 3a. The authors can therefore assume the following lithiation process: very small (several nm) Li nanocrystals adjust their orientation and fuse to form larger crystals. As the larger crystals continue to grow and come into contact with each other, grain boundaries form. Indeed, this “critical” size of Li grains is in good agreement with the observations in [17]. To identify the nature of the point defect, the authors show in Figure 3b two versions of one HRTEM image. The line profiles of the image intensity of the graphene lattice in Figure 3b are extracted as shown in Figure 3c. As can be seen, one carbon atom is missing in the graphene lattice. Therefore, the point defect as a carbon vacancy is easily identified. The authors point out that in the TEM experiments, the concentration of vacancies in the graphene-Li system is much higher than in isolated graphene sheets [20]. DFT calculations confirm that vacancies in graphene can act as seed cells for the growth of lithium crystals (Figure 3d). A similar behavior has also been reported for the interaction of lithium in graphene with double vacancies [21]. It should be noted that the adsorption of lithium atoms at a single vacancy is energetically more favorable than at double vacancies.
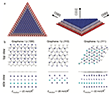
Next, Figure 4a shows another atomically resolved HRTEM image of a small faceted Li crystal. Figure 4b is a zoomed-in view of the boxed region in Figure 4a, which clearly shows the Li lattice with six-fold symmetry. The distance between two nearest atomic columns is 3.1 Å, which is in good agreement with the previous result [17]. Each Li atomic column has 6 nearest neighbors. Figure 4c shows the fcc model with ABC stacking (positions A, B, C are shaded differently) between two graphene layers (gray). Figure 4d shows the Fourier transform of the raw data of Figure 4a (Li and graphene reflections are present). The spatial frequencies of the 1st to 3rd order reflections of graphene are indicated by three red half rings, while those of the 2nd and 3rd order of Li are indicated by two blue solid half rings. Based on the measurement of the first visible reflection in the experimental Li image with a spatial frequency of 6.3 nm−1, the lattice constant of the Li crystals between the graphene layers was estimated to be 4.4 Å, which is consistent with the value reported in the literature for fcc-Li [21].
In Figure 5a, the authors identify the nature of the foreign atoms by acquiring local electron energy-loss spectroscopy (EELS) data before and after lithiation. When comparing the two spectra, a Li signal is seen after lithiation, confirming the insertion of Li ions during the lithiation. Moreover, a weak oxygen K-edge signal is observed after lithiation, indicating that the heavier foreign atoms introduced during lithiation are oxygen. To understand the interaction of the foreign O atoms with fcc Li between graphene sheets, DFT calculations were performed with O atoms placed between graphene but outside the Li crystal. The result suggests that the O atoms prefer to be “sucked” into the Li crystal instead of being located between the graphene layer and the Li slab. This behavior is similar to C atoms implanted into Cu foils [22]. Calculations [21] indeed show that, of the two possible interstitial positions in an fcc crystal [23], O atoms prefer the octahedral one because its formation energy is lower than the other. The atomic model with an O atom in the octahedral interstitial position, shown in the side and top views in Figure 5b, is used as the input data for the subsequent HRTEM simulation. Figure 5c presents experimental HRTEM images recorded with different defoci containing the defects (top row) and the corresponding simulated images (bottom row).
To rationalize the observations, Figure 6a shows calculations of the surface energies of different possible surfaces of fcc Li crystals with low indices, which are in good agreement with previously reported values [24]. Interestingly, the calculations indicated that the (111) surface is the highest in energy. To explain this contradiction, one should also consider the energy of the interface between graphene and the Li crystal, which can partially compensate for the energy penalty upon surface creation (with regard to the infinite slab). The calculation setup is presented in Figure 6b. The interface between the (111) Li surface and graphene is energetically most favorable, and the sum of the interface and surface energies is the smallest, indicating that this type of interface must form. The edges should then be represented by (100) surfaces, which in turn have the lowest formation energy, so that the whole picture is consistent.
Figure 7a shows a series of HRTEM images recorded during another delithiation process inside bilayer graphene (the graphene lattice is removed, and the orientation maps of Li are overlaid). The edges of the Li phase in each image are marked by the white dotted lines. The image series clearly shows that the Li phase shrinks (as the colored area decreases), which is associated with the transfer of the Li ions back into the electrolyte during the delithiation. The edges of the Li phase are jagged, and no facets can be found, although most of the Li phase is still crystalline. Figure 7b shows magnified views of the boxed areas in Figure 7a, and it is clearly seen that many O atoms (a few examples are marked by the arrows) gather at the edges of the Li crystal, forming a ca. 1 nm thick oxidized surface layer. It should be noted that there is already LixOy material in the system as a residual from the last cycle; some examples are marked by the arrows in Figure 7a.
Resource: Li, Y., Börrnert, F., Ghorbani-Asl, M., Biskupek, J., Zhang, X., Zhang, Y., Bresser, D., Krasheninnikov, A. V., Kaiser, U. (2024). In Situ TEM Investigation of the Lithiation and Delithiation Process Between Graphene Sheets in the Presence of Atomic Defects. Advanced Functional Materials. DOI: 10.1002/adfm.202406034
-
Masias, A., Marcicki, J., & Paxton, W. A. (2021). Opportunities and challenges of lithium-ion batteries in automotive applications. ACS Energy Letters, 6(2), 621-630. https://doi.org/10.1021/acsenergylett.0c02529
-
Edge, J. S., O’Kane, S., Prosser, R., Kirkaldy, N. D., Patel, A. N., Hales, A., Ghosh, A., Ai, W., Chen, J., Yang, J., Li, S., Pang, M.-C., Diaz, L. B., Tomaszewska, A., Marzook, M. W., Radhakrishnan, K. N., Wang, H., Patel, Y., Wu, B., & Offer, G. J. (2021). Lithium-ion battery degradation: what you need to know. Physical Chemistry Chemical Physics, 23(14), 8200-8221. https://doi.org/10.1039/D0CP06521J
-
Yang, X. G., Leng, Y., Zhang, G., Ge, S., & Wang, C. Y. (2017). Modeling of lithium plating induced aging of lithium-ion batteries: Transition from linear to nonlinear aging. Journal of Power Sources, 360, 28-40. https://doi.org/10.1016/j.jpowsour.2017.05.121
-
Gilbert, J. A., Shkrob, I. A., & Abraham, D. P. (2017). Transition metal dissolution, ion migration, electrocatalytic reduction and capacity loss in lithium-ion full cells. Journal of the Electrochemical Society, 164(2), A389. https://doi.org/10.1149/2.0161702jes
-
Zhan, C., Wu, T., Lu, J., & Amine, K. (2018). Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes – a critical review. Energy & Environmental Science, 11(2), 243-257. https://doi.org/10.1039/C7EE03122J
-
Soto, F. A., Marzouk, A., El-Mellouhi, F., & Balbuena, P. B. (2018). Understanding ionic diffusion through SEI components for lithium-ion and sodium-ion batteries: Insights from first-principles calculations. Chemistry of Materials, 30(10), 3315-3322. https://doi.org/10.1021/acs.chemmater.7b05077
-
Liu, X. M., & Arnold, C. B. (2020). Effects of current density on defect-induced capacity fade through localized plating in lithium-ion batteries. Journal of the Electrochemical Society, 167(13), 130519. https://doi.org/10.1149/1945-7111/abb58c
-
Jia, Y., Liu, B., Hong, Z., Yin, S., Finegan, D. P., & Xu, J. (2020). Safety issues of defective lithium-ion batteries: identification and risk evaluation. Journal of Materials Chemistry A, 8(25), 12472-12484. https://doi.org/10.1039/D0TA03550A
-
Giuli, G., Trapananti, A., Mueller, F., Bresser, D., d’Acapito, F., & Passerini, S. (2015). Insights into the effect of iron and cobalt doping on the structure of nanosized ZnO. Inorganic Chemistry, 54(19), 9393-9400. https://doi.org/10.1021/acs.inorgchem.5b01386
-
Asenbauer, J., Hoefling, A., Indris, S., Tübke, J., Passerini, S., & Bresser, D. (2020). Mechanistic insights into the lithiation and delithiation of iron-doped zinc oxide: the nucleation site model. ACS Applied Materials & Interfaces, 12(7), 8206-8218. https://doi.org/10.1021/acsami.9b20494
-
Rahman, M. M., Chen, W. Y., Mu, L., Xu, Z., Xiao, Z., Li, M., Bai, X.-M., & Lin, F. (2020). Defect and structural evolution under high-energy ion irradiation informs battery materials design for extreme environments. Nature Communications, 11(1), 4548. https://doi.org/10.1038/s41467-020-18417-y
-
Cui, Y., Yao, H., Zhang, J., Zhang, T., Wang, Y., Hong, L., Xian, K., Xu, B., Zhang, S., Peng, J., Wei, Z., Gao, F., & Hou, J. (2019). Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nature Communications, 10(1), 2515. https://doi.org/10.1038/s41467-019-10525-9
-
Vu, N. H., Arunkumar, P., Im, J. C., Ngo, D. T., Le, H. T., Park, C. J., & Im, W. B. (2017). Effect of synthesis temperature on the structural defects of integrated spinel-layered Li1.2Mn0.75Ni0.25O2+δ: a strategy to develop high-capacity cathode materials for Li-ion batteries. Journal of Materials Chemistry A, 5(30), 15730-15742. https://doi.org/10.1039/C7TA04866C
-
Reynaud, M., Serrano-Sevillano, J., & Casas-Cabanas, M. (2023). Imperfect battery materials: a closer look at the role of defects in electrochemical performance. Chemistry of Materials, 35(9), 3345-3363. https://doi.org/10.1021/acs.chemmater.2c02905
-
Asenbauer, J., Eisenmann, T., Kuenzel, M., Kazzazi, A., Chen, Z., & Bresser, D. (2020). The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustainable Energy & Fuels, 4(11), 5387-5416. https://doi.org/10.1039/D0SE00175A
-
Kühne, M., Paolucci, F., Popovic, J., Ostrovsky, P. M., Maier, J., & Smet, J. H. (2017). Ultrafast lithium diffusion in bilayer graphene. Nature Nanotechnology, 12(9), 895-900. https://doi.org/10.1038/nnano.2017.116
-
Kühne, M., Börrnert, F., Fecher, S., Ghorbani-Asl, M., Biskupek, J., Samuelis, D., Krasheninnikov, A. V., Kaiser, U., & Smet, J. H. (2018). Reversible superdense ordering of lithium between two graphene sheets. Nature, 564(7735), 234-239. https://doi.org/10.1038/s41586-018-0764-z
-
Linck, M., Hartel, P., Uhlemann, S., Kahl, F., Müller, H., Zach, J., Haider, M., Niestadt, M., Bischoff, M., Biskupek, J., Lee, Z., Lehnert, T., Börrnert, F., Rose, H. & Kaiser, U. (2016). Chromatic aberration correction for atomic resolution TEM imaging from 20 to 80 kV. Physical Review Letters, 117(7), 076101. https://doi.org/10.1103/PhysRevLett.117.076101
-
Lehtinen, O., Komsa, H. P., Pulkin, A., Whitwick, M. B., Chen, M. W., Lehnert, T., Mohn, M. J., Yazyev, O. V., Kis, A., Kaiser, U., & Krasheninnikov, A. V. (2015). Atomic scale microstructure and properties of Se-deficient two-dimensional MoSe2. ACS Nano, 9(3), 3274-3283. https://doi.org/10.1021/acsnano.5b00137
-
Meyer, J. C., Eder, F., Kurasch, S., Skakalova, V., Kotakoski, J., Park, H. J., Roth, S., Chuvilin, A., Eyhusen, S., Benner, G., Krasheninnikov, A. V., & Kaiser, U. (2012). Accurate measurement of electron beam induced displacement cross sections for single-layer graphene. Physical Review Letters, 108(19), 196102. https://doi.org/10.1103/PhysRevLett.108.196102
-
Zhang, X., Ghorbani-Asl, M., Zhang, Y., & Krasheninnikov, A. V. (2023). Quasi-2D FCC lithium crystals inside defective bi-layer graphene: insights from first-principles calculations. Materials Today Energy, 34, 101293. https://doi.org/10.1016/j.mtener.2023.101293
-
Riikonen, S., Krasheninnikov, A. V., Halonen, L., & Nieminen, R. M. (2012). The role of stable and mobile carbon adspecies in copper-promoted graphene growth. The Journal of Physical Chemistry C, 116(9), 5802-5809. https://doi.org/10.1021/jp211951b
-
Hu, X., Björkman, T., Lipsanen, H., Sun, L., & Krasheninnikov, A. V. (2015). Solubility of boron, carbon, and nitrogen in transition metals: getting insight into trends from first-principles calculations. The Journal of Physical Chemistry Letters, 6(16), 3263-3268. https://doi.org/10.1021/acs.jpclett.5b01388
-
Liu, M., Kutana, A., Liu, Y., & Yakobson, B. I. (2014). First-principles studies of Li nucleation on graphene. The Journal of Physical Chemistry Letters, 5(7), 1225-1229. https://doi.org/10.1021/jz500236g