Bottom-up formation of robust gold carbide
March 16, 2015 - In Joule heating experiments up to 1000 K of graphene decorated with gold particles in the electron microscope, scientists discovered unknown crystals with quadratic arrangements which had a larger lattice constant than pure gold clusters. They identified the material that has never been synthesized up until now as gold carbide. For their analysis they combined aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM) with density-functional theory calculations (DFT). The results were published today in the open-access Nature journal Scientific reports.
The existence of a gold carbide was first demonstrated in 1900 when Matthew & Watters reported on the highly explosive substance gold acetylide Au2C2 [1]. Today, there is already a research field called organogold chemistry [2] which deals with the structures that contain carbon-gold bonds. However, inorganic crystalline gold-carbon compounds have not yet been found experimentally [3]. This is not surprising because the solubility of carbon in gold is nearly 0 and the only above-mentioned potential crystalline gold carbide turned out to be extremely unstable [4]. Only single gold carbide clusters could be synthesized, but they are not electrically neutral and contain only a small number of atoms.
The successful formation of robust gold carbide was also made possible by the 2D material graphene. "We used graphene as the substrate for the gold atoms and in-situ heating element by applying a voltage. Furthermore both graphene and the hydrocarbons on graphene could have served as a supplier of carbon for the chemical reaction," said Prof. Ute Kaiser, director of the SALVE project, who lead the study. "In addition, the property of the electron beam also affects the interaction of carbon and gold. The combination of these special features of the experiment now enabled us to synthesize a new gold-carbon compound through a bottom-up process atom-by-atom in-situ in the electron microscope for the first time."
The work with carbon nanostructures in the TEM has enormously benefited from the development of aberration correction [5], since atomic resolution was made possible with low electron energies, in which low-dimensional carbon in materials remain much more stable. Due to the high mechanical stability, graphene can now be used as sample support in electron microscopy [6] and because of its high thermal conductivity, graphene is stable at least in the range from room temperature (RT) up to 2000 K [7].
The use of two-dimensional materials for in-situ experiments in the TEM, however, places a high demand on sample preparation. The aim was to allow imaging of heated Au and C atoms with atomic resolution in real time. A mechanically exfoliated graphene flake was transferred to a Si/SiN membrane with open windows and gold contacts, resulting in an electrically contacted and completely free-standing graphene layer in TEM compatible geometry. The gold nano-islands were previously ex-situ deposited by thermal evaporation.
HRTEM image analysis
In order to make the gold particles visible, the graphene layer must show deviations from the perfect crystalline structure. The diffusion of gold atoms occurs already at room temperature [8, 9] and leads to the migration of the particles. Liquid gold droplets form above a certain temperature, which than start to vaporize [7, 10, 11]. The diffusion barrier for gold on graphene is extremely low, theoretical estimates yielded only 0.05 eV [12, 13]. Therefore, single gold atoms are only visible when they are trapped by grain boundaries, edge reconstructions or vacancies [14]. This is also confirmed by recently published DFT calculations [13, 15, 16]. In addition, the influence of the electron beam has to be considered, as discussed by Urban & Seeger [17] and Banhart [18], which additionally increases the migration of the gold atoms.
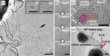
The AC-HRTEM images offered unique insights into the continuous interaction of Au and C. In the image analysis, the scientists found that clusters with square lattice structures were formed on amorphous carbon. However, in contrast to pure gold, the observed clusters had a considerably larger internal atomic distance of 3.35 Å (Fig. 1aii). Only one example could be found where the atomic distance was the same as in bulk gold (2.88 Å, Fig. 1ai, [19]). However, it was not possible to determine experimentally the kind of structure and composition of the unknown particles since light elements between gold atoms were not recognizable under these imaging conditions. The scientists therefore named the unknown structure Au-X for the time being. From the experiments it was only known that X was an element with a low atomic number. The following video shows the formation of this structure. More videos are available as supporting information.
Density functional theory calculations
In order to unravel the unknown element's nature, the scientists simulated the atomic distances of all possible elements and structural arrangements by means of density functional theory (DFT). Only the elements C, N and O yielded a lattice parameter with sufficient agreement with the observations (Table 1). The calculated values for e. g. Si differed by 11 - 25% depending on the structure ('zinc-blende', 'rock-salt' and 'fluorite') (Table 1) and could thus be excluded. Si could also be excluded purely experimentally by Electron Energy Loss Spectroscopy (EELS), as the respective peak was absent.
The least deviation from the experimental results is shown by the NaCl structure of AuC, AuN and AuO. However, a systematic deviation of DFT calculations with the generalized gradient approximation (GGA) from the actual observations has to be considered [20, 21]. So, for example, the DFT value for Au is 2.4% higher than the experimental value. Taking into account this systematic deviation, the best agreement is obtained for AuC in ZnS structure.
Further analyzes were possible by comparing the experimentally observed orientation of the crystals with the simulation results. The cuboids were observed only with a surface termination of (100). In the case of the ZnS structure, the DFT simulation predicted precisely this surface termination, which means that this termination should be observed more frequently than (110) and (111) (Fig. 1). In addition, the simulation showed that it should be more energetically favorable if the crystal is present with an Au atomic layer on the surface, which also coincides with the experimental observations. In contrast, for the NaCl structure the (100) direction is the energetically most unfavorable. Thus, the NaCl structure could be excluded for the Au-X compound.
This result was additionally confirmed by further analyzes. (1) The mixing of the carbon matrix and gold was observed at the atomic level in the experiment at high temperatures (Fig. 2). (2) According to the literature, gold oxides and gold nitrides should have a different stoichiometry and crystal structure. (3) It was found that the cuboids were stable up to a temperature of about 1000 K. This is above the decomposition temperature of gold oxide and gold nitride [22, 23].
The AuC formed by a bottom-up process is metastable but surprisingly robust under electron irradiation and at high temperatures. The electronic structure of AuC on graphene is almost identical to that of the isolated material. Therefore, only a very small interaction occurs between graphene and the underlying substrate. The new material has a bulk modulus of 141 GPA which is close to the value of bulk Au. Thus, as also other transition metal carbides, its properties are very attractive for the industry. [...]"
Highlighted Topics
Resource: Westenfelder, B., Biskupek, J., Meyer, J. C., Kurasch, S., Lin, X., Scholz, F., Gross, A. & Kaiser, U. A. (2015). Bottom-up formation of robust gold carbide. Scientific reports, 5: 8891, doi: 10.1038/srep08891, [PDF], see also the supporting information PDF, supporting information Video 1, Video 2, Video 3, Video 4 and Video 5.
Mathews, J. A., & Watters, L. L. (1900). The carbide of gold. Journal of the American Chemical Society, 22: 108-111, doi: 10.1021/ja02040a010
Parish, R. V. (1997). Organogold chemistry: I structure and synthesis. Gold Bulletin, 30: 3-12, doi: 10.1007/BF03214751
Cohen, Y., Bernshtein, V., Armon, E., Bekkerman, A., & Kolodney, E. (2011). Formation and emission of gold and silver carbide cluster ions in a single C−. The Journal of chemical physics, 134: 124701, doi: :10.1063/1.3561317
Okamoto, H., & Massalski, T. B. (1984). The Au-C (gold-carbon) system. Journal of Phase Equilibria, 5: 378-379, doi: 10.1007/BF02872953
Haider, M., Uhlemann, S., Schwan, E., Rose, H., Kabius, B., & Urban, K. (1998). Electron microscopy image enhanced. Nature, 392: 768-769, doi: 10.1038/33823
Ross, F. M. (2007). In situ transmission electron microscopy. In Science of Microscopy, 445-534. Springer New York, doi: 10.1007/978-0-387-49762-4_6
Westenfelder, B., Meyer, J. C., Biskupek, J., Algara-Siller, G., Lechner, L. G., Kusterer, J., Kaiser, U. A., Krill III, C. E., Kohn, E. & Scholz, F. (2011). Graphene-based sample supports for in situ high-resolution TEM electrical investigations. Journal of Physics D, 44: 055502, doi: 10.1088/0022-3727/44/5/055502
Bovin, J. O., Wallenberg, R., & Smith, D. J. (1985). Imaging of atomic clouds outside the surfaces of gold crystals by electron microscopy. Nature, 317: 47-49, doi: 10.1038/317047a0
Iijima, S., & Ichihashi, T. (1985). Motion of surface atoms on small gold particles revealed by HREM with real-time VTR system. Japanese Journal of Applied Physics, 24: L125, doi: 10.1143/JJAP.24.L125
Westenfelder, B., Meyer, J. C., Biskupek, J., Kurasch, S., Scholz, F., Krill III, C. E., & Kaiser, U. (2011). Transformations of carbon adsorbates on graphene substrates under extreme heat. Nano letters, 11: 5123-5127, doi: 10.1021/nl203224z
Barnard, A. S., Young, N. P., Kirkland, A. I., Van Huis, M. A., & Xu, H. (2009). Nanogold: a quantitative phase map. ACS nano, 3: 1431-1436, doi: 10.1021/nn900220k
Jensen, P., Blase, X., & Ordejón, P. (2004). First principles study of gold adsorption and diffusion on graphite. Surface Science, 564: 173-178, doi: 10.1016/j.susc.2004.06.188
Hardcastle, T. P., Seabourne, C. R., Zan, R., Brydson, R. M. D., Bangert, U., Ramasse, Q. M., Novoselov, K. S., & Scott, A. J. (2013). Mobile metal adatoms on single layer, bilayer, and trilayer graphene: An ab initio DFT study with van der Waals corrections correlated with electron microscopy data. Physical Review B, 87: 195430, doi: 10.1103/PhysRevB.87.195430
Cretu, O., Krasheninnikov, A. V., Rodríguez-Manzo, J. A., Sun, L., Nieminen, R. M., & Banhart, F. (2010). Migration and localization of metal atoms on strained graphene. Physical review letters, 105: 196102, doi: 10.1103/PhysRevLett.105.196102
Malola, S., Häkkinen, H., & Koskinen, P. (2009). Gold in graphene: in-plane adsorption and diffusion. Applied Physics Letters, 94: 043106, doi: 10.1063/1.3075216
Tang, Y., Yang, Z., & Dai, X. (2011). Trapping of metal atoms in the defects on graphene. The Journal of chemical physics, 135: 224704, doi: 10.1063/1.3666849
Urban, K., & Seeger, A. (1974). Radiation-induced diffusion of point-defects during low-temperature electron irradiation. Philosophical Magazine, 30: 1395-1418, doi: 10.1080/14786437408207289
Banhart, F. (1999). Irradiation effects in carbon nanostructures. Reports on Progress in Physics, 62: 1181, doi: 10.1088/0034-4885/62/8/201
Wang, J., Wang, G., & Zhao, J. (2002). Density-functional study of Au n (n= 2–2 0) clusters: Lowest-energy structures and electronic properties. Physical Review B, 66: 035418, doi: 10.1103/PhysRevB.66.035418
Lischka, M., & Groß, A. (2002). Hydrogen adsorption on an open metal surface: H2/Pd (210). Physical Review B, 65: 075420, doi: 10.1103/PhysRevB.65.075420
Lischka, M., Mosch, C., & Groß, A. (2007). Tuning catalytic properties of bimetallic surfaces: Oxygen adsorption on pseudomorphic Pt/Ru overlayers. Electrochimica acta, 52: 2219-2228, doi: 10.1016/j.electacta.2006.03.113
Krishnamurthy, S., Montalti, M., Wardle, M. G., Shaw, M. J., Briddon, P. R., Svensson, K., Hunt, M. R. C. & Šiller, L. (2004). Nitrogen ion irradiation of Au (110): Photoemission spectroscopy and possible crystal structures of gold nitride. Physical Review B, 70: 045414, doi: 10.1103/PhysRevB.70.045414
Maya, L., Paranthaman, M., Thundat, T., & Bauer, M. L. (1996). Gold oxide as precursor to gold/silica nanocomposites. Journal of Vacuum Science & Technology B, 14: 15-21, doi: 10.1116/1.589020
Biskupek, J., Hartel, P., Haider, M., & Kaiser, U. (2012). Effects of residual aberrations explored on single-walled carbon nanotubes. Ultramicroscopy, 116: 1-7, doi: 10.1016/j.ultramic.2012.03.008
Perdew, J. P., Burke, K., & Ernzerhof, M. (1996). Generalized gradient approximation made simple. Physical review letters, 77: 3865, doi: 10.1103/PhysRevLett.77.3865
Kresse, G., & Furthmüller, J. (1996). Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical review B, 54: 11169, doi: 10.1103/PhysRevB.54.11169
Kresse, G., & Furthmüller, J. (1996). Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 6: 15-50, doi: 10.1016/0927-0256(96)00008-0
Blöchl, P. E. (1994). Projector augmented-wave method. Physical Review B, 50: 17953, doi: 10.1103/PhysRevB.50.17953
Kresse, G., & Joubert, D. (1999). From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 59: 1758, doi: 10.1103/PhysRevB.59.1758
Birch, F. (1978). Finite strain isotherm and velocities for single‐crystal and polycrystalline NaCl at high pressures and 300° K. Journal of Geophysical Research: Solid Earth, 83: 1257-1268, doi: 10.1029/JB083iB03p01257