Benefits and limitations of low-kV macromolecular imaging of frozen-hydrated biological samples
February 23, 2016 - Researchers have long been trying to develop technologies that improve the resolution for biological samples embedded in ice ("Cryo-Microscopy"). High-brightness electron gun, zero loss energy filtering [1-3] and more recently the physical phase plates [4, 5] were tested in this regard, but none of these techniques has so far reached significant improvement in macromolecular imaging; however direct electron detection did and is now standard in high-resolution cryo electron microscopy. Could low-voltage high-resolution electron microscopy, which enabled imaging of 2-dimensional materials with atomic resolution, lead also here to a quantum leap?
Several recent studies have shown that quasi-atomic imaging of biological macromolecules is no longer pure fantasy, as e.g. ref. [6]. A comprehensive overview of the available but not yet commonly used novel methods has pointed to the use of low energy electrons as an interesting approach [7]. Low-voltages are crucial for atomic-resolution imaging of 2D materials [8, 9] below their knock-on damage thresholds. This electron beam damage mechanism occurs when incident electrons hit an atom, so that it can be released from its chemical bond [10, 11]. It was moreover reported that low-voltage microscopy allowed the in-situ analysis of dynamic behavior such as cis-trans isomerization of organic molecules [12]. Thus one might expect that low voltages are also useful for macromolecular imaging of biological samples embedded in ice. Any significant improvement in sample resolution for biological samples would possibly trigger a revolution in biology.
Using the C>S-corrected SALVE II TEM, a study was now conducted in which the advantages and limitations of low voltage Cryo-electron microscopy have been studied experimentally. The scientists involved are researchers from the University of Heidelberg, the company Carl Zeiss, and the University of Ulm. The study finds that the increased ionization at low voltages for standard samples (about 50 nm thick) cannot be compensated by the increased contrast. It should be marked that by using thin samples, the use of low voltages, however, might actually lead to the resolution enhancement in cryo microscopy, which however would request a CC/CS-corrected TEM. Corresponding experiments are now possible, after the SALVE project created with the acceptance of the SALVE III mircoscope the possibilities for low voltage CC/CS-corrected TEM in April 2016.
The data was collected at 20 kV, 40 kV and 80 kV with the SALVE II microscope at Carl Zeiss Microscopy Oberkochen, Germany and at 60 kV with the Zeiss Libra 200 Kronos TEM at Heidelberg University, Germany. Each of the microscope features a second-order-corrected, in-column energy filter and an imaging CS corrector, developed by CEOS Company, Heidelberg, Germany. Test samples of this study were tobacco mosaic virus (TMV) particles embedded in ice.
Increased amplitude contrast at low kV
The experiments with the biological samples embedded in ice used in this study show increasing amplitude contrast for decreasing electron energy. Here, the amplitude contrast increases faster than the phase contrast contribution [14]. The contrast determined from zero-loss filtered images is 4.3% at 80 kV, 6.0% at 60 kV, 8.5% at 40 kV and 14% at 20 kV (i.e. the amplitude contrast increases by a factor of 2, when the electron energy is reduced from 80 keV to 40 keV and nearly another factor of two when the electron energy is reduced from 40 to 20 keV, see Fig. 2 A-D). To determine the contrast enhancement, which is due to the zero-loss filtering, the researchers compared the values with data obtained from unfiltered images of TMV embedded in amorphous ice (Fig. 2, I-L). Thereby effects by chromatic aberration could be neglected, since these only affect high resolution images and are irrelevant for the studied images at low resolution. [1] The contrast of the TMV particles for the unfiltered images was 2.1% at 80 kV, 3.4% at 60 kV, 3.8% at 40 kV and 6.8% at 20 kV (Fig 2, I-L). The data showed that decreasing electron energy results qualitatively in the same contrast enhancement as in zero-loss filtered images. The decrease of amplitude contrast with increasing electron energy can, to first order, be described by a function proportional to the scattering cross-section σ of the electrons in matter, which is proportional to 1/U (where U is the electron accelerating voltage) [14] (Fig. 3).
Analysis of structural damage as a function of electron dose and electron energy
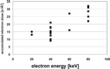
The tolerable dose of accumulated electrons increases with electron energy (Fig. 4). The data points represent the accumulated electron dose at which the first layer of TMV disappears. The results are characteristic for radiation damage due to ionization of the molecules.
Signal/noise ratio at low electron energies: sample thickness is a limiting factor
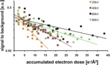
The crucial question is therefore what signal can be obtained at a certain electron energy of the sample, before the structure is destroyed. To investigate this problem, the scientists analyzed on the basis of the recorded zero-loss filtered data the signal/background ratio (SBR) of a layer TMV depending on the accumulated electron dose and electron energy (Fig. 5). The data shows that under the chosen experimental conditions the best SBR is obtained for a beam energy of 60 keV. The 80 keV data points show an almost equally high SBR, the SBR at 40 keV and 20 kV is significantly smaller.
Possible future sample preparation is directed to very thin substrates of carbon material, e.g. enclosing the material into a graphene sandwich [19]. Further research is needed to show whether such samples can be made without affecting the structure of single small biological molecules. Moreover, it has to be tested, to what extent reducing the electron dose per time may decrease the radiation damage. Another approach to improve the resolution of HRTEM data of thicker samples, especially at low electron energy < 60 kV, is the use of inelastically scattered electrons for high-resolution imaging. With the use of the low-voltage chromatic aberration-corrected TEM (SALVE III TEM) thicker samples may be mapped in three dimensions [20, 21].
This new study offers an important look at which options are available to increase the resolution in cryo-microscopy. "Rasmus Schröder and his team have experimentally accessed two important quantities that determine the resolution in macromolecular imaging of vitrified samples: radiation damage and contrast." Kaiser says. "We will now further pursue the two most promising approaches for a resolution improvement, advancing specimen preparation and usage of chromatic aberration correction, both on a state-of-the-art technological level in the frame of the SALVE III project. This can only be reached by a close cooperation between electron microscopists working in materials and life sciences."
Highlighted Topics
Tolerable dose and amplitude contrast at lower voltages
Imaging of biological samples in TEM
Direct measurement of amplitude contrast
Resource: Majorovits, E., Angert, I., Kaiser, U., & Schröder, R. R. (2016). Benefits and limitations of low-kV macromolecular imaging of frozen-hydrated biological samples. Biophysical Journal, 110: 776-784, doi: 10.1016/j.bpj.2016.01.023, [PDF].
Langmore, J. P., & Smith, M. F. (1992). Quantitative energy-filtered electron microscopy of biological molecules in ice. Ultramicroscopy, 46: 349-373, doi: 10.1016/0304-3991(92)90024-E
Angert, I., Majorovits, E., & Schröder, R. R. (2000). Zero-loss image formation and modified contrast transfer theory in EFTEM. Ultramicroscopy, 81: 203-222, doi: 10.1016/S0304-3991(99)00190-4
Yonekura, K., Braunfeld, M. B., Maki-Yonekura, S., & Agard, D. A. (2006). Electron energy filtering significantly improves amplitude contrast of frozen-hydrated protein at 300 kV. Journal of structural biology, 156: 524-536, doi: 10.1016/j.jsb.2006.07.016
Murata, K., Liu, X., Danev, R., Jakana, J., Schmid, M. F., King, J., Nagayama, K., & Chiu, W. (2010). Zernike phase contrast cryo-electron microscopy and tomography for structure determination at nanometer and sub-nanometer resolutions. Structure, 18: 903-912, doi: 10.1016/j.str.2010.06.006
Danev, R., Buijsse, B., Khoshouei, M., Plitzko, J. M., & Baumeister, W. (2014). Volta potential phase plate for in-focus phase contrast transmission electron microscopy. Proceedings of the National Academy of Sciences, 111: 15635-15640, doi: 10.1073/pnas.1418377111
Bartesaghi, A., Merk, A., Banerjee, S., Matthies, D., Wu, X., Milne, J. L., & Subramaniam, S. (2015). 2.2 Å resolution cryo-EM structure of β-galactosidase in complex with a cell-permeant inhibitor. Science, 348: 1147-1151, doi: 10.1126/science.aab1576
Schröder, R. R. (2015). Advances in electron microscopy: A qualitative view of instrumentation development for macromolecular imaging and tomography. Archives of biochemistry and biophysics, 581: 25-38, doi: 10.1016/j.abb.2015.05.010
Kaiser, U., Biskupek, J., Meyer, J. C., Leschner, J., Lechner, L., Rose, H., Stöger-Pollach, M., Khlobystov, A. N., Hartel, P., Müller, H., Haider, M., Eyhusen, S., & Benner, G. (2011). Transmission electron microscopy at 20 kV for imaging and spectroscopy. Ultramicroscopy, 111: 1239-1246, doi: 10.1016/j.ultramic.2011.03.012
SALVE III Project. http://www.salve-project.de/, accessed January 29, 2016.
Kotakoski, J., Krasheninnikov, A. V., Kaiser, U., & Meyer, J. C. (2011). From point defects in graphene to two-dimensional amorphous carbon. Physical Review Letters, 106: 105505, doi: 10.1103/PhysRevLett.106.105505
Girit, C. Ö., Meyer, J. C., Erni, R., Rossell, M. D., Kisielowski, C., Yang, L., Park, C.-H., Crommie, M. F., Cohen, M. L., Louie, S. G., & Zettl, A. (2009). Graphene at the edge: stability and dynamics. Science, 323: 1705-1708, doi: 10.1126/science.1166999
Nakamura, E. (2013). Movies of molecular motions and reactions: the single‐molecule, real‐time transmission electron microscope imaging technique. Angewandte Chemie International Edition, 52: 236-252, doi: 10.1002/anie.201205693
Rose, H. (2010). Theoretical aspects of image formation in the aberration-corrected electron microscope. Ultramicroscopy, 110: 488-499, doi: 10.1016/j.ultramic.2009.10.003
Hayashida, M., Kawasaki, T., Kimura, Y., & Takai, Y. (2006). Estimation of suitable condition for observing copper–phthalocyanine crystalline film by transmission electron microscopy. Nuclear Instruments and Methods in Physics Research Section B, 248: 273-278, doi: 10.1016/j.nimb.2006.04.168
Lee, Z., Meyer, J. C., Rose, H., & Kaiser, U. (2012). Optimum HRTEM image contrast at 20kV and 80kV—Exemplified by graphene. Ultramicroscopy, 112 39-46, doi: 10.1016/j.ultramic.2011.10.009
Meyer, J. C., Kisielowski, C., Erni, R., Rossell, M. D., Crommie, M. F., & Zettl, A. (2008). Direct imaging of lattice atoms and topological defects in graphene membranes. Nano letters, 8: 3582-3586, doi: 10.1021/nl801386m
Bell, D. C., Russo, C. J., & Kolmykov, D. V. (2012). 40keV atomic resolution TEM. Ultramicroscopy, 114: 31-37, doi: 10.1016/j.ultramic.2011.12.001
Haider, M., Uhlemann, S., Schwan, E., Rose, H., Kabius, B., & Urban, K. (1998). Electron microscopy image enhanced. Nature, 392: 768-769, doi: 10.1038/33823
Algara-Siller, G., Kurasch, S., Sedighi, M., Lehtinen, O., & Kaiser, U. (2013). The pristine atomic structure of MoS2 monolayer protected from electron radiation damage by graphene. Applied Physics Letters, 103: 203107, doi: 10.1063/1.4830036
Wacker, I. U., Röder, I. V., Rudolf, R., Hillmer, S., Kabius, B., Hofhaus, G., & Schröder, R. (2010). Studying membrane topology of the neuromuscular junction by electron tomography of thick resin slices in a CS/CC-corrected TEM. Microscopy and Microanalysis, 16: 972-973, doi: 10.1017/S1431927610056217
Baudoin, J. P., Jinschek, J. R., Boothroyd, C. B., Dunin-Borkowski, R. E., & de Jonge, N. (2013). Chromatic aberration-corrected tilt series transmission electron microscopy of nanoparticles in a whole mount macrophage cell. Microscopy and Microanalysis, 19: 814-820, doi: 0.1017/S1431927613001475
Linck, M., Hartel, P., Uhlemann, S., Kahl, F., Müller, H., Zach, J., Haider, M., Niestadt, M., Bischoff, M., Biskupek, J., Lee, Z., Lehnert, T., Börrnert, F., Rose, H. H. & Kaiser, U. A. (2016). Chromatic Aberration Correction for Atomic Resolution TEM Imaging from 20 to 80 kV. Physical Review Letters, 117: 076101, doi: 10.1103/PhysRevLett.117.076101