Stationary Atoms to Control Solidification in Liquid Metal Nanoparticles
December 9, 2025 – Researchers from Ulm University (Germany) and the University of Nottingham (United Kingdom) have discovered that stationary atoms play a crucial role in the solidification process of liquid metal nanoparticles. Using aberration-corrected low-voltage TEM, they demonstrated that metal atoms bound to graphene vacancies can keep liquid platinum super-cooled more than 1000 °C below the bulk solidification temperature, enabling unprecedented control over crystallization dynamics at the atomic scale.
The formation of solids is crucial for various natural phenomena such as mineralization, ice crystallization, and protein folding, as well as for technological processes like metallurgy and the pharmaceutical industry. Fundamental principles of interatomic bonding and the interactions of atoms with their environment regulate this process and often exhibit unusual thermodynamics and complex kinetics that differ from classical processes. [1,2,3] Real-time microscopy techniques allow the direct observation of nucleation and growth in the solid phase and are therefore powerful tools for elucidating crystallization mechanisms. [4,5] Previous studies have shown that even the solidification of pure metals—the simplest case in which atoms of the same type combine to form a lattice—proceeds via complex atomic mechanisms. Early nucleation stages in gold, rhenium, and iron have been investigated using TEM imaging. It was shown that a critical number of atoms must converge in a two-step process to form a stable crystal nucleus. [6]
Continuing from ref. 6, the present work found that the proportion of stationary atoms plays a crucial role in the solidification process. This includes the formation of metastable crystal nuclei as well as the coexistence of solid, amorphous, and crystalline phases within the same nanoparticle. Stationary atoms surrounding a liquid particle can lead to a super-cooled state of the molten metal, more than 1000 °C below the solidification temperature of the bulk material. All these processes occur simultaneously in the same material and are strongly influenced by the local particle environment, as demonstrated by atomically resolved, continuous observations of the solidification dynamics over a wide temperature range of 20–800 °C. To visualize atomic dynamics—such as the liquid-to-solid transition—systematic analyses of HRTEM image sequences are required.
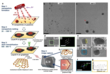
The authors use single-layer graphene (SLG) on a heated TEM grid as a support for metal atoms, which are deposited directly onto it by magnetron sputtering (step 1, Fig. 1a). The metal atoms self-organize into clusters whose size depends on the surface density of the metal atoms, the concentration and type of surface defects, and the temperature. [7,8] The method used here requires no reagents or solvents and thus ensures that platinum, palladium, and gold are investigated in their natural metallic form in HRTEM experiments.
By utilizing micro-electro-mechanical system (MEMS) heated chip technology in combination with the high thermal conductivity of graphene, the authors controlled the temperature of the sample within a range of 20–800 °C. Once the temperature has stabilized and the sample's drift caused by thermal expansion or contraction has subsided, atomic resolution in the images can be maintained (step 2, Fig. 1a). Due to graphene's thinness and high thermal conductivity, beam-induced heating is not a concern in the sample. [9] As the temperature changes, the authors use CC/CS-corrected low-voltage TEM imaging at 60 or 80 kV electron beam to observe transformations in individual nanoparticles with high-resolution. [10]
The dual nature of electrons also allows the use of their wave nature to generate images through the phase contrast mechanism (Fig. 1a), as well as harness their momentum, enabling them to act as projectiles on carbon atoms in graphene. This interaction, termed the direct knock-on effect, can displace carbon atoms from the graphene lattice and create additional vacancies (Step 3, Fig. 1a). Platinum and other metals act as catalysts and lower the energy barrier, [11] as confirmed by density functional theory (DFT) calculations. Defects in graphene serve as effective binding sites for metal atoms through the formation of covalent M-C bonds. The metal nanoparticles are then irradiated and melted. With an increasing number of defects in the graphene, more metal atoms bind to the graphene. The rate of this process is determined by the rate of the direct collision effect, which is proportional to the electron beam flux. [12]
The measurements show that significant changes in the number of stationary metal atoms in liquid Pt nanoparticles occur only at very high flux densities of approximately 109 electrons/(nm² s). The type of metal plays an important role: for Au, even this flux is insufficient, while for Pd, a moderate flux of ~106 electrons/(nm² s) triggers rapid changes that are difficult to track in real time. In molten Pt, the high flux density allows the control of the number and position of stationary metal atoms bound to vacancies in graphene by irradiating a selected liquid nanoparticle with a condensed electron beam (step 3, Fig. 1a). This makes it possible to investigate the influence of the number of stationary atoms on the solidification of Pt while the sample temperature is gradually lowered and HRTEM images of specific nanoparticles are sequentially acquired (step 4, Fig. 1a).
HRTEM images show that metal atoms on SLG aggregate at room temperature to form 2–3 nm nanoparticles alongside the randomly present amorphous carbon on the graphene (Fig. 1b). Due to the low contrast of the underlying carbon, the metal atoms on the graphene are clearly visible and often form a lattice resembling the face-centered cubic (fcc) lattice of solid platinum (Fig. 1d). Theoretical modeling of platinum nanoparticles predicts that Pt(111) is the most stable on graphene, [2,13] suggesting that the nanoparticles are initially kinetically stabilized.
Heating to 800 °C causes the sharp atomic contrast in the solid nanoparticles to soften into a diffuse contrast and develop smooth, convex menisci, as expected for a liquid nanodroplet, indicating melting (Fig. 1e). The FFT reflections of the Pt metal lattice disappear, with the only lattice contrast corresponding to the underlying graphene visible through the layer of liquid metal (Fig. 1f). However, the authors found that the atomic contrast of some isolated Pt atoms remains sharp and unaffected by the heat. This suggests that their positions remain constant at least during image acquisition (approximately 1 s per image) or longer, as they are bound to vacancies (Figs. 1e and 1h). In some cases, the second graphene layer, composed of annealed amorphous carbon, envelops the nanodroplets, maintaining a spacing of 0.4 nm (Figs. 1e–4). Similar behavior was observed for molten Pd and Au nanoparticles.
The HRTEM image sequence shows the displacement of carbon atoms in contact with platinum. This leads to an increasing number of vacancies in the graphene with increasing flux, comparable to other metals. [11,14,15,16] If the defects are located along the zigzag edge of the second graphene layer surrounding a molten platinum particle, the stationary platinum atoms arrange themselves in a chain spaced approximately 0.25 nm apart (Figs. 1e and 1g).
At a moderate electron beam flux density of 106 e−/(nm²s), as is typically used for HRTEM imaging, the authors observe the melting and solidification of nanoparticles that remain unaffected by the electron beam. Upon heating to 800 °C, all nanoparticles adhere to a carbon layer adsorbed on SLG (from randomly present amorphous carbon), while individual metal atoms, as expected due to strong covalent metal-carbon bonds, [17] are embedded in vacancies. In the solid state, the metal atoms of a nanoparticle form a lattice that exhibits strong contrast in the HRTEM image for all three metals [18] and completely obscures the graphene lattice (Figs. 1b and 1d). However, if the temperature rises above 600 °C, the metal contrast disappears, the nanoparticle becomes transparent, and the underlying graphene lattice becomes visible. This allows the solid and liquid states to be clearly distinguished (Figs. 1c and 1e for Pt).
The bonding energy of Pt with monovacancy and divacancy defects is very high, 7.26 and 7.21 eV, respectively. [17] Therefore, in the temperature range of 20 to 800 °C that was investigated, the Pt atoms bonded to the vacancy defects cannot acquire enough kinetic energy for their displacement from either the thermal motion of the liquid or the direct momentum transfer from the electron beam (the maximum energy that can be transferred from the 80 kV electron beam to the Pt atoms is 0.97 eV). As a result, the Pt atoms associated with the defects are firmly bonded to the graphene, which keeps them stationary during the time it takes to capture images, allowing them to display sharp atomic contrast in HRTEM images.
The authors were able to show that irradiating a single molten particle with a high flux of the 80 kV electron beam—by focusing the beam on the particle for a few seconds (Fig. 2a)—results in the liquid nanoparticle being surrounded by a ring of fixed platinum atoms (Fig. 2b). Further high-flux irradiation can completely solidify the particle. Liquid nanoparticles can indeed be solidified in a single step by irradiation with a high-energy electron beam and thus directly transformed into a crystalline phase without a temperature change (Fig. 2c). This electron beam-induced solidification occurs in molten Pt nanoparticles of varying sizes, forming face-centered cubic (fcc) lattices with different orientations. Remarkably, the nanoparticle remains liquid even under intense electron irradiation when completely encased in a second graphene layer. These observations demonstrate that controlling the environment of molten Pt nanoparticles with an electron beam allows for effective manipulation of their state.
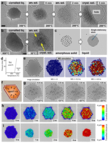
Using MEMS-heated chip technology, the authors gradually lowered the temperature from 20 to 800 °C and observed the changes in the nanoparticles using HRTEM. Surprisingly, the solidification dynamics of molten platinum showed considerable variation, even within the same field of view. This resulted in different solidification temperatures and mechanisms, influenced by the local environment (Fig. 3a–c). The solidification temperatures were generally below the melting temperatures, which is consistent with the hysteresis predicted by calculations using the embedded atomic method (EAM) (Fig. 3a, inset). In the simple case of homogeneous nucleation, the crystalline phase coexists with the liquid phase within the same nanoparticle. This metastable state undergoes dissolution and recrystallization within minutes, with a critical nucleation size of approximately 2 nm (Fig. 3d). Stationary atoms in the liquid favor crystallization from the center outwards, with the crystal faces gradually spreading across the entire nanoparticle (Fig. 3d). However, molten nanoparticles enclosed by a ring of stationary Pt atoms behave differently. While unenclosed Pt nanoparticles crystallize at around 500 °C, as evidenced by the appearance of a Pt-fcc lattice contrast (Fig. 3b), the enclosed particles remain liquid down to significantly lower temperatures and fluctuate between liquid and amorphous solid states (Fig. 3c, e).
The observations indicate that nearly every liquid nanoparticle contains a small number of stationary Pt atoms bound to defects in the graphene, while other atoms remain mobile within the liquid. Theoretically, the crystallization of liquid nanodroplets exhibits a significant activation barrier, leading to melt-solidification hysteresis (Fig. 3a, inset). [19,20] Nucleation begins in the center or at the edges and spreads throughout the entire volume of the nanodroplet. [3,19,20] The experiments show that liquid Pt is in equilibrium with a crystallization nucleus when a small number of stationary atoms cannot disrupt the process. This is confirmed by a time-domain HRTEM image of platinum at 500 °C (Fig. 3d). A crystal lattice of 10–12 atomic layers, which forms directly from the liquid (as in frame 4 s and 13 s in Fig. 3d), is metastable and melts and regrows several times before the nanoparticle completely solidifies (frame 26 s).
High-intensity electron beam irradiation allows for the increase of defects in graphene around a specific liquid nanoparticle. This enables the targeted enhancement of the number of stationary atoms and the investigation of their influence on the dynamics of liquid metal solidification. The most unexpected phenomena occur in liquid Pt nanodroplets when they are surrounded by a ring of stationary Pt atoms. The liquid core enclosed by stationary atoms remains liquid up to 200–350 °C, indicating a high activation barrier for crystal nucleation in this unusual metallic state. For example, crystallization from the periphery is inhibited by the restricted Pt-Pt distances resulting from the free bonds of the graphene. These distances do not correspond to the lattice constants of a face-centered cubic (fcc) crystal lattice. Consequently, the liquid-crystal nucleation pathways observed in free liquids are blocked in the enclosed state. This leads to experimental observations of a hindered nucleation process, which manifests itself in fluctuations between the amorphous and liquid states of platinum (Fig. 3e).
Remarkably, the cores of the enclosed nanoparticles remain in a super-cooled liquid state even at 350 °C, as evidenced by the absence of a distinct atomic Pt contrast. They only solidify into an amorphous phase at around 200 °C (Fig. 4a). This amorphous solid phase can spontaneously or through electron beam irradiation transition to a crystalline state, leading to stresses and fractures in the underlying graphene (Fig. 4a, last image). However, larger, encapsulated nanoparticles appear to be able to retain both crystalline and amorphous phases during solidification without stressing the graphene (Fig. 4b, right image).
Upon cooling to approximately 200 °C, the Pt-Pt bond energy in super-cooled liquid nano-droplets exceeds their kinetic energy, leading to the solidification of the enclosed liquid metal into an amorphous state. Amorphous metal nanoparticles are known to be significantly less stable than crystalline ones. [2,20] The experiments show, however, that their crystallization can only occur if the enclosure ruptures, either spontaneously at 200 °C (Fig. 4a, last image) or by electron beam excitation at 30 °C. Larger enclosed liquid particles with a diameter of approximately 20 nm, on the other hand, can partially crystallize without the enclosure rupturing. In this case, a crystalline phase forms in the center, while the edges remain amorphous (Fig. 4b). The distribution of the structural stress of the amorphous solid phase over a larger number of Pt atoms appears to have a stabilizing effect on the nanoparticles.
To distinguish randomly localized and delocalized (mobile) atoms in an amorphous solid and a liquid nanoparticle, respectively, the authors performed HRTEM image simulations with stationary, randomly positioned Pt atoms and with Pt atoms delocalized within the nanoparticle volume (Fig. 4c, left and right images). The simulated images for liquid metal show that the graphene lattice as well as individual stationary Pt atoms are clearly visible through a layer of liquid metal using HRTEM (Fig. 4c, right). Likewise, the simulated image of the amorphous solid (Fig. 4c, left) agrees well with the experimental image (Fig. 4a, second image), further confirming the ability of HRTEM imaging to clearly distinguish between the two states. Furthermore, there is a thermodynamic factor that limits the crystallization of the enclosed nanoparticles. This factor is due to the higher density of solid Pt compared to liquid Pt, which leads to a negative volume change (Fig. 4d).
Unlike free, molten nanoparticles, the volume of the enclosed liquid cannot be easily adjusted. This would require either breaking the Pt-C bond in the enclosed region or stretching the graphene layer, whose in-plane elastic modulus is 1 TPa [21] – both highly inefficient methods. This presents an additional hurdle for crystallization (Fig. 4f).
Furthermore, the authors performed molecular dynamics (MD) simulations to investigate the temporal evolution of an enclosed, liquid Pt nanoparticle with a diameter of approximately 3 nm at constant temperature and its solidification dynamics as the temperature decreased. For comparison, similar MD simulations were performed to determine the differences in the temporal atomic dynamics of an unenclosed, liquid Pt nanoparticle on graphene at constant temperature and its solidification dynamics as the temperature decreased. The results showed that the Pt atoms in the center of the enclosed nanodroplet underwent significant shifts (red atoms, Fig. 4e), while the atoms at the edges appeared to be localized due to strong bonds with carbon atoms (blue atoms, Fig. 4e). Additionally, the simulated HRTEM images from the MD trajectories showed good agreement with experimental observations. The mobile Pt atoms in the center are not visible in HRTEM, thus revealing the graphene lattice, while stationary atoms at the periphery exhibit sharp atomic contrast (Fig. 4e, left image). The HRTEM images simulated directly from the MD models correlated well with experimental data for an enclosed liquid with some defects in the basal graphene layer (Fig. 4g). As the temperature decreased, the number of stationary atoms increased, leading to the formation of an amorphous solid nanoparticle (Fig. 4g, last image). This is consistent with the HRTEM experiments (Fig. 4a, second image). These results demonstrate that HRTEM imaging can effectively distinguish between the liquid and amorphous solid states of metal nanoparticles, thus enabling the observation of the temperature transition with atomic precision.
This study focuses on platinum on carbon due to its technological importance. However, the here shown methodology was also applied to palladium and gold nanoparticles on graphene, which were produced using the same procedure and have a size distribution of 2–5 nm. Similar to platinum, Pd and Au are liquid at 750 °C, losing the atomic structure of the metal lattice. Some metal atoms bind to vacancies. In contrast to platinum, however, Au has a lower binding energy to the graphitic carbon (2.97 eV for Au vs. 7.41 eV for Pt). This limits the controllability of the number of vacancies and thus also the number of stationary Au atoms. Even a high electron beam flux of 80 kV cannot therefore generate enough stationary atoms to influence the solidification process of gold (Fig. 5a).
Compared to platinum, the valence electron shell of palladium (Pd) remains unchanged, thus preserving the considerable binding energy of palladium and carbon (5.46 eV). [17] This allows for similar defect formation rates to those of Pt. However, due to the lower atomic mass of palladium, it receives almost twice as much kinetic energy from the electron beam as platinum (1.78 eV for Pd vs. 0.97 eV for Pt). The increased kinetic energy transferred to palladium by the incident electron enhances its atomic mobility. Therefore, the crystallization process cannot be sufficiently interrupted by the presence of stationary Pd atoms to keep its rate comparable to the HRTEM image acquisition rate. The measurements show that even a moderate electron beam flux induces crystallization in Pd nanoparticles without changing the temperature, making the onset of this process unpredictable (Fig. 5b).
Resource: Christopher Leist, Sadegh Ghaderzadeh, Emerson C. Kohlrausch, Johannes Biskupek, Luke T. Norman, Ilya Popov, Jesum Alves Fernandes, Ute Kaiser, Elena Besley & Andrei N. Khlobystov (2025). Stationary Atoms in Liquid Metals and Their Role in Solidification Mechanisms. ACS Nano. DOI: 10.1021/acsnano.5c05437
Kayhani, K. et al. (2010). Surface effect on the coalescence of Pt clusters: A molecular dynamics study. Appl. Surf. Sci. 256, 6982. https://doi.org/10.1016/j.apsusc.2010.05.010
Lee, S. H. et al. (2008). Phase stability of Pt nanoclusters and the effect of a (0001) graphite surface through molecular dynamics simulation. Surf. Sci. 602, 1433. https://doi.org/10.1016/j.susc.2008.01.033
Nam, H. S. et al. (2002). Formation of an icosahedral structure during the freezing of gold nanoclusters: Surface-induced mechanism. Phys. Rev. Lett. 89, 275502. https://doi.org/10.1103/PhysRevLett.89.275502
Hussein, H. E. M. et al. (2018). Tracking metal electrodeposition dynamics from nucleation and growth of a single atom to a crystalline nanoparticle. ACS Nano 12, 7388. https://doi.org/10.1021/acsnano.8b04089
Shen, X. et al. (2018). Deconvolution of octahedral Pt₃Ni nanoparticle growth pathway from in situ characterizations. Nat. Commun. 9, 4485. https://doi.org/10.1038/s41467-018-06900-z
Cao, K. et al. (2020). Atomic mechanism of metal crystal nucleus formation in a single-walled carbon nanotube. Nat. Chem. 12, 921. https://doi.org/10.1038/s41557-020-0538-9
Kohlrausch, E. C. et al. (2021). A high-throughput, solvent-free method for dispersing metal atoms directly onto supports. J. Mater. Chem. A 9, 26676. https://doi.org/10.1039/D1TA08372D
Popov, I. et al. (2023). Chemical kinetics of metal single atom and nanocluster formation on surfaces: An example of Pt on hexagonal boron nitride. Nano Lett. 23, 8006. https://doi.org/10.1021/acs.nanolett.3c01968
Börner, P., Kaiser, U. & Lehtinen, O. (2016). Evidence against a universal electron-beam-induced virtual temperature in graphene. Phys. Rev. B 93, 134104. https://doi.org/10.1103/PhysRevB.93.134104
Linck, M. et al. (2016). Chromatic aberration correction for atomic resolution TEM imaging from 20 to 80 kV. Phys. Rev. Lett. 117, 076101. https://doi.org/10.1103/PhysRevLett.117.076101
Cao, K. et al. (2018). Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts. Nat. Commun. 9, 3382. https://doi.org/10.1038/s41467-018-05831-z
Skowron, S. T. et al. (2017). Chemical reactions of molecules promoted and simultaneously imaged by the electron beam in transmission electron microscopy. Acc. Chem. Res. 50, 1797. https://doi.org/10.1021/acs.accounts.7b00078
Ellaby, T. et al. (2018). Ideal versus real: Simulated annealing of experimentally derived and geometric platinum nanoparticles. J. Phys. Condens. Matter 30, 155301. https://doi.org/10.1088/1361-648X/aab251
Zoberbier, T. et al. (2012). Interactions and reactions of transition metal clusters with the interior of single-walled carbon nanotubes imaged at the atomic scale. J. Am. Chem. Soc. 134, 3073. https://doi.org/10.1021/ja208746z
Lebedeva, I. V. et al. (2014). The atomistic mechanism of carbon nanotube cutting catalyzed by nickel under an electron beam. Nanoscale 6, 14877. https://doi.org/10.1039/C4NR05006A
Zoberbier, T. et al. (2016). Investigation of the interactions and bonding between carbon and group VIII metals at the atomic scale. Small 12, 1649. https://doi.org/10.1002/smll.201502210
Jovanović, A. Z. et al. (2021). Reactivity screening of single atoms on modified graphene surface: From formation and scaling relations to catalytic activity. Adv. Mater. Interfaces 8, 2001814. https://doi.org/10.1002/admi.202001814
Williams, D. B. & Carter, C. B. (2009). Transmission electron microscopy: A textbook for materials science. Springer: New York. https://doi.org/10.1007/978-0-387-76501-3
Gafner, S. L. et al. (2012). Computer analysis of some thermodynamic properties of platinum nanoclusters by melting–crystallization processes. J. Comput. Theor. Nanosci. 9, 993. https://doi.org/10.1166/jctn.2012.2131
Hou, M. (2017). Solid-liquid and liquid-solid transitions in metal nanoparticles. Phys. Chem. Chem. Phys. 19, 5994. https://doi.org/10.1039/c6cp08606c
Lee, J. U., Yoon, D. & Cheong, H. (2012). Estimation of Young's modulus of graphene by Raman spectroscopy. Nano Lett. 12, 4444. https://doi.org/10.1021/nl301073q